Assisting a Large Pharmaceutical Company with Their eClinical Documentation Initiative
Business Challenges
Clinical trial protocols are complex by nature as well as inconsistent across the biopharmaceutical industry. This makes clinical trial implementation and reporting difficult for investigators, regulatory authorities, and patients. To address these issues, TransCelerate BioPharma Inc. has developed a Common Protocol Template (CPT) with their industry members – a model clinical trial protocol template containing a common structure and model language that utilizes libraries to facilitate reuse of content by therapeutic area and patient population. The CPT is meant to be a first step towards establishing an electronic protocol.
Our client, a large pharmaceutical company and TransCelerate member, decided to adopt the CPT, but wanted to extend its design principles and concepts to apply to the Statistical Analysis Plan (SAP) and Clinical Study Report (CSR) Body templates as well. Since the CPT was developed as a standalone document, the client’s eClinical documentation initiative would require extending the core CPT functionality and developing a content design for documents across the clinical continuum.
The ArborSys Solution
Initially, ArborSys collaborated closely with the client to perform a high-level organizational readiness review, needs assessment, and business case/roadmap for a broad-based Structured Content Management (SCM) implementation. Although the client agreed on the considerable value of SCM, they decided to postpone making any decisions on a technology platform to align the investment with their broader strategy and roadmap.
The client still wanted to make progress with the structuring and governing of their content, however, as a stepping stone towards the authoring and review process changes required to adopt SCM. In order to assist the client move forward with their eClinical documentation initiative, ArborSys proposed to continue with the key tasks that are required to be completed for an SCM initiative that were technology agnostic, and leverage some of the core concepts for “smart templates” coming from the work done for the TransCelerate CPT.
To that end, ArborSys assisted the client with the following:
- Analysis of their clinical content (Protocol, SAP, CSR Body)
- This included a mapping of the client’s existing protocol template to the CPT, from a both structural and content perspective
- Development of the content design and information model
- The clinical content was broken up into “components” of information and assigned metadata and reuse policies for structuring the content
- Design and implementation of content libraries for key reusable content
- Libraries were developed for instructional text and standard text; additional libraries for therapeutic area-specific text were planned for future release
- Standard content creation and governance processes were aligned with those already established by the client’s internal metadata repository (MDR) team, to enable MDR team support of the clinical content
- Implementation of “smart templates” using the same concepts and approaches developed for the CPT
- The CPT core functionality, all of which is available via Microsoft Word, was extended according to the client’s specific requirements (eg, enabling content reuse across documents, managing library content in SharePoint Lists/Libraries)
- Development of new processes for authoring, reviewing, and finalizing the content/
documents- This included governance processes and guidelines
- Conduct of a small pilot with the clinical document types to validate the change management and governance processes required for the SCM program
- Feedback, lessons learned, and recommendations were compiled
- Production rollout of the “smart templates” to the global organization
Value Delivered
This interim approach facilitates incremental process and cultural changes and allows the business to take the steps towards SCM implementation without having to purchase a full SCM technology platform upfront. Trying to enact key process changes in parallel with the adoption of a new and fairly complex technology platform can be a very difficult and lengthy process.
The content design and development of the “smart templates” was able to be done in parallel with the client continuing their SCM technology assessment and platform decision-making. The benefit of ArborSys’s technology-agnostic approach is that the key information design and process changes are addressed upfront, and the outputs can be migrated easily into any SCM technology platform. Creating documents with the “smart templates” also enabled the client to gain experience authoring in a structured manner – content was written in components that could be reused in other document types. The ability to tag components for reuse across documents was a significant extension of the CPT’s core functionality that ArborSys provided for the client. This shift in the content creation paradigm is an important change management concept for authors to embrace so that they can “write with purpose”. Although the client started with the protocol, SAP, and CSR Body, a component-based approach would allow for content reuse across the clinical document continuum, as illustrated below.
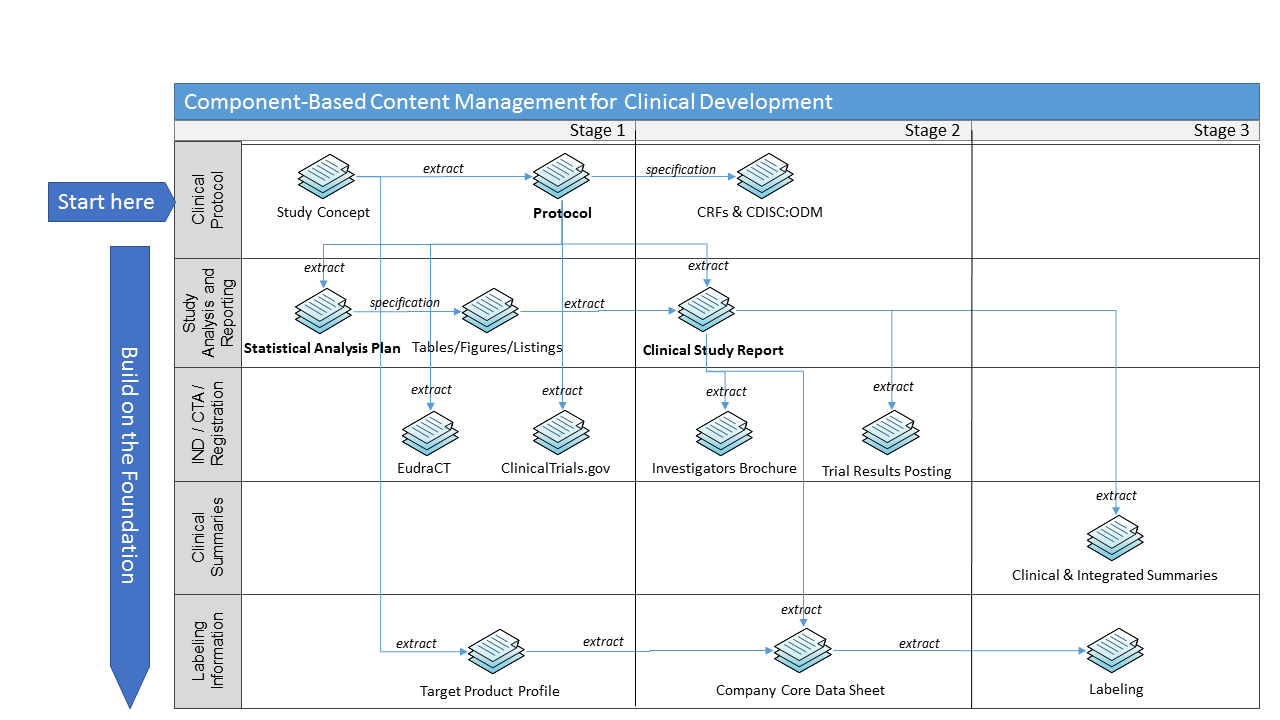
Furthermore, by building on the TransCelerate CPT’s core structure and design principles, the client was able to align with an emerging industry “standard” for the clinical trial protocol, making implementation and reporting by investigators, regulatory authorities, and patients more efficient and effective.
Download PDF of this Case Study
Want to learn more?
For more information about this case study or any of our services, contact us by email: sales@arborsys.com
