Optimizing FDA Form 1572 Process and Activity Management
Business Challenges
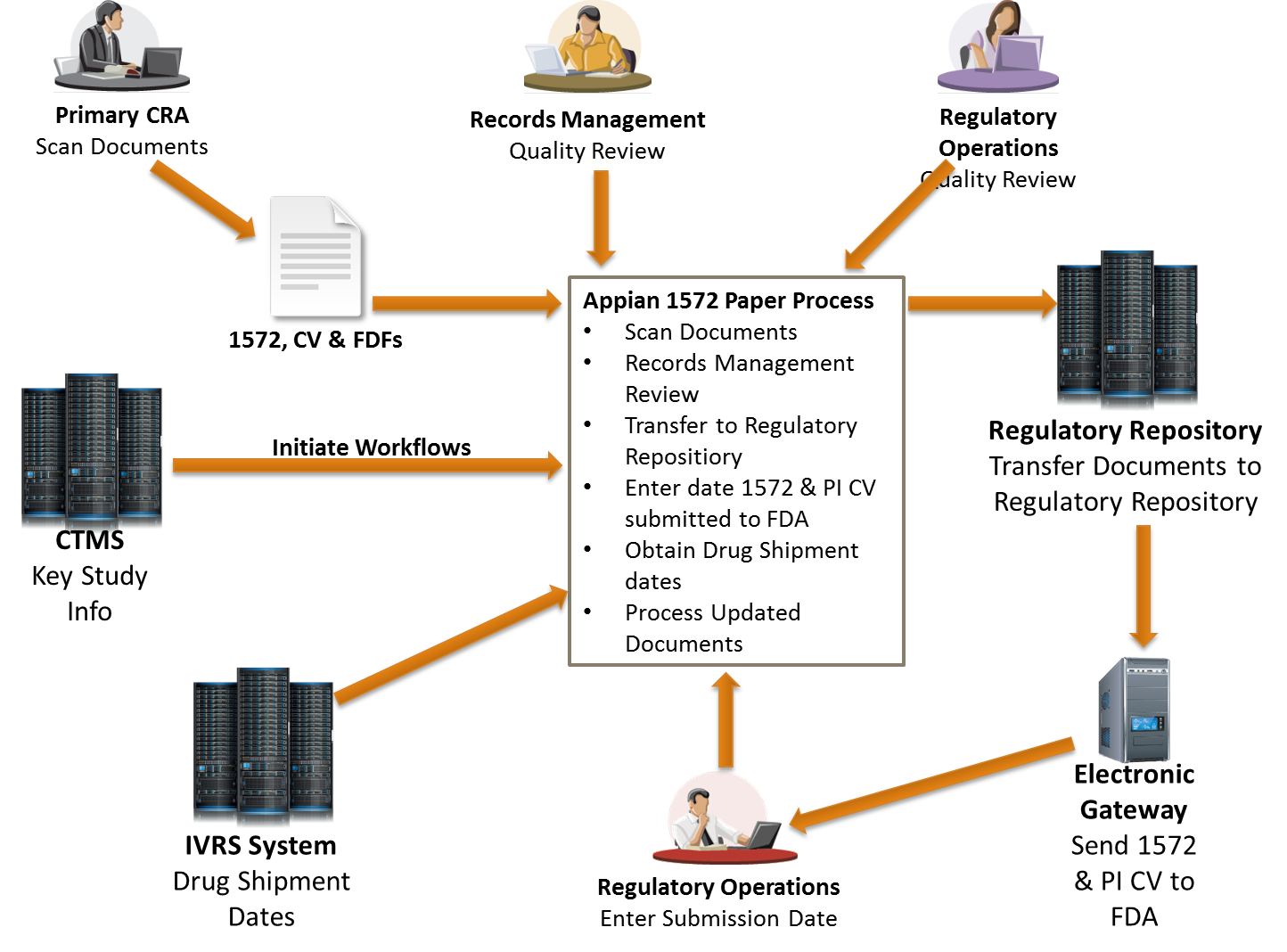
Investigator document submissions represent a critical milestone, including the FDA Form 1572, Primary Investigator (PI) Curriculum Vitae (CV), and a Financial Disclosure Form (FDF) for each investigator on the study. The 1572 and PI CV must be submitted within 30 days of the Drug Shipment Date, and compliance with this task using manual processes often results in delays. ArborSys consultants worked side-by-side with business and technology associates of a global pharmaceutical company to develop an electronic workflow for approval and submission of these investigator documents.
The ArborSys Solution
The SRD (Submission Regulatory Documents) 1572 Project Team focused on building quality into the process from the start and improving on-going regulatory document compliance. Business process experts were gathered from across the organization. Using ArborSys's structured approach, the existing processes were first documented and then new streamlined, harmonized processes were developed.
Utilizing the client's business process management (BPM) software, Appian, team members were able to quickly adapt new automated workflows to increase the timeliness of document submissions and the accuracy of study documents. The application monitors every step in the process in real-time, and makes individuals accountable for each task they accept. Additionally, the application will import data from the Clinical Trial Management System (CTMS) using an on-going data feed and then transfers documents to the regulatory repository after they have been processed.
Key performance metrics also were developed including: number of users and locations, connectivity and performance, document quality, and required rework.
The application is currently be used by personnel in charge of document submission and management activities (e.g., Clinical Research Associate, Clinical Project Leader, Regulatory Operations, etc.) for 2 clinical studies that include over 200 study sites.
Value Delivered
The new application improved quality and regulatory compliance, while lowering the risk of non-compliance by providing traceability of documents for a study, and by creating a new standardized and automated process for the FDA 1572 forms, CVs, and FDFs delivering:
- Automated workflows for uploading and approving the study documents
- A basis for evaluating the Submission document preparation readiness
- Process visibility to ensure compliance in a timely manner
- Automated transfer of final approved documents into the client's regulatory repository
- Tracking and reporting of process status
- Metrics to monitor compliance with timelines as defined in the respective processes
Major business goals achieved were:
- Reduced risk of regulatory compliance issues
- Facilitated collaboration with external partners
- Improved investigator selection, monitoring, and relationship
- Improved flexibility, speed, and productivity
Want to learn more?
For more information about this case study or any of our services, contact us by email: sales@arborsys.com
