Optimizing the Quality Review and Remediation of Clinical Trial Management System Data
Business Challenges
ArborSys consultants worked side-by-side with business and technology associates of a global pharmaceutical company on a multi-year initiative to improve end-to-end clinical development through business process management (BPM), innovation, and technology. The project was initiated to optimize the quality review and remediation of Clinical Trial Management System (CTMS) data. The project’s goal was to ensure that study data from the CTMS are accurate, complete, consistent, and reported in a timely manner using an efficient and standardized process to meet the mandated regulatory reporting deadlines.
The ArborSys Solution

The Project Team focused on building quality into the new process from the start. The existing manual processes were initially created as a way to check the quality and completeness of data in a legacy CTMS built by the client. Over time, the manual processes also were applied to two additional CTMS. To begin the process improvement activities, subject matter experts of the manual processes were gathered from across the organization. Using ArborSys’ structured approach, the existing processes were documented and then new harmonized processes aligned to technology were developed.
The new application was designed using a data integration tool, Informatica PowerCenter, and a BPM tool, Appian, to automate the process of performing quality reviews (completion, update, and verification) of selected study and regulatory data. The data are extracted from source systems and placed in a temporary staging area (e.g., a clinical data hub). Staged data are then evaluated by Informatica against a predefined set of data quality rules, with a record added to the application’s data mart for each data error. Study profile data also are added to the database. The completed database contains records of all data quality errors as well as profiles of all active studies.
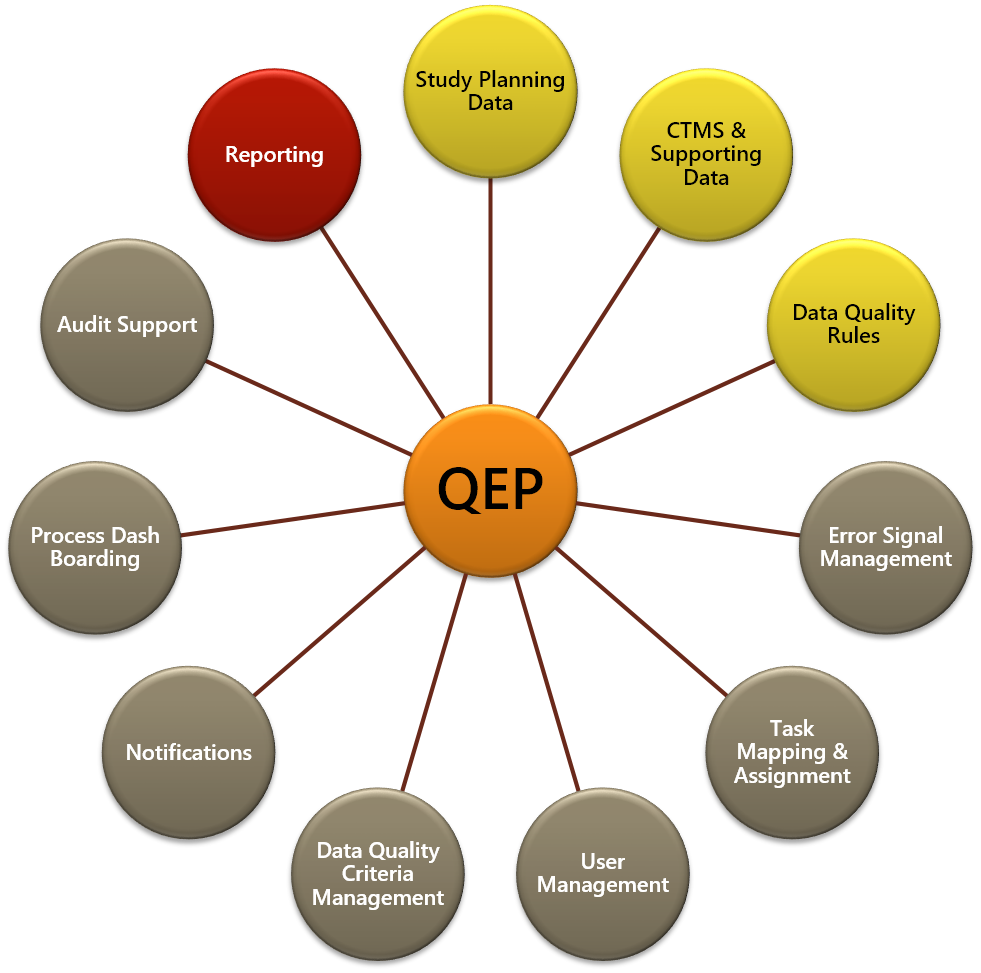
Appian evaluates the data error signal records and assigns them as correction tasks according to a predefined workflow. Upon detection, Appian assigns the error(s) to the appropriate CTMS user for correction and notifies that person of the assignment via email. In the even that an error is detected for a critical study – e.g., the study’s data are to be included in an upcoming regulatory report – an additional email is sent to the CTMS user’s manager to ensure immediate action by the user. Process data also are made available for reporting and dash boarding to allow senior management to track the progress of CTMS data quality reviews and corrections.
This Application serves as:
- An aid to ensure timely remediation of data errors that are reported by the interface.
- A basis for evaluating the data error signals and tasks for remediating the errors.
- A source of metrics and reports that can be generated to monitor compliance with quality review timelines.
Value Delivered
Major business objectives were achieved with the realization of the following:
- Reduced risk of regulatory non-compliance by improving the quality and completeness of CTMS data.
- Facilitated collaboration through more efficient, automated, and standardized processes.
- Improved flexibility, speed, and productivity to achieve accurate CTMS data on a daily basis compliance (instead of a monthly basis via the manual processes).
Want to learn more?
For more information about this case study or any of our services, contact us by email: sales@arborsys.com
