Delivering a Roadmap for Achieving Strategic Advantage from Knowledge in Pharmaceutical R&D
Business Challenges
Pharmaceutical companies must make large investments in very long R&D programs to be successful. The development effort to bring a new drug to market averages 7 10 years with full lifecycle costs ranging between $800M-$1B. Numerous studies indicate that 20-30% of this time and cost is consumed by the effort to find and react to queries on past outcomes to meet various audits and reviews that occur along the way. The data, information, and knowledge created are vast in their size, scope, and diverse number of internal and external parties that generate and consume them.
Pharmaceutical Development owns a major component of this work that starts with the early stage drug candidates from discovery to delivery and support in manufacturing of a successful program. This group requires the ability to quickly react to the evolving needs of Clinical Operations, Regulatory Authorities, and Manufacturing over many active trials over many years. Too often, past knowledge is too hard to find and effort is wasted repeating work to meet the needs of their customers. One company conducted a detailed analysis and uncovered over 1200 instances when data, information, or knowledge was generated or consumed over the life of the Pharmaceutical Development process. The result was that R&D Leadership recognized the significant value of improving how knowledge is management. A decision was made to determine the roadmap for moving forward.
The ArborSys Solution
ArborSys helped our client deliver on long-term strategy to transform how they create, curate, and consume their knowledge assets. We employed our business-driven approach to create their business capability architecture, determine the critical to quality business needs, and then map these needs to the client’s existing architecture. The business capabilities required further enhancement to determine the knowledge capabilities required to transform their business activities to support the creation, curation, and consumption of knowledge assets.
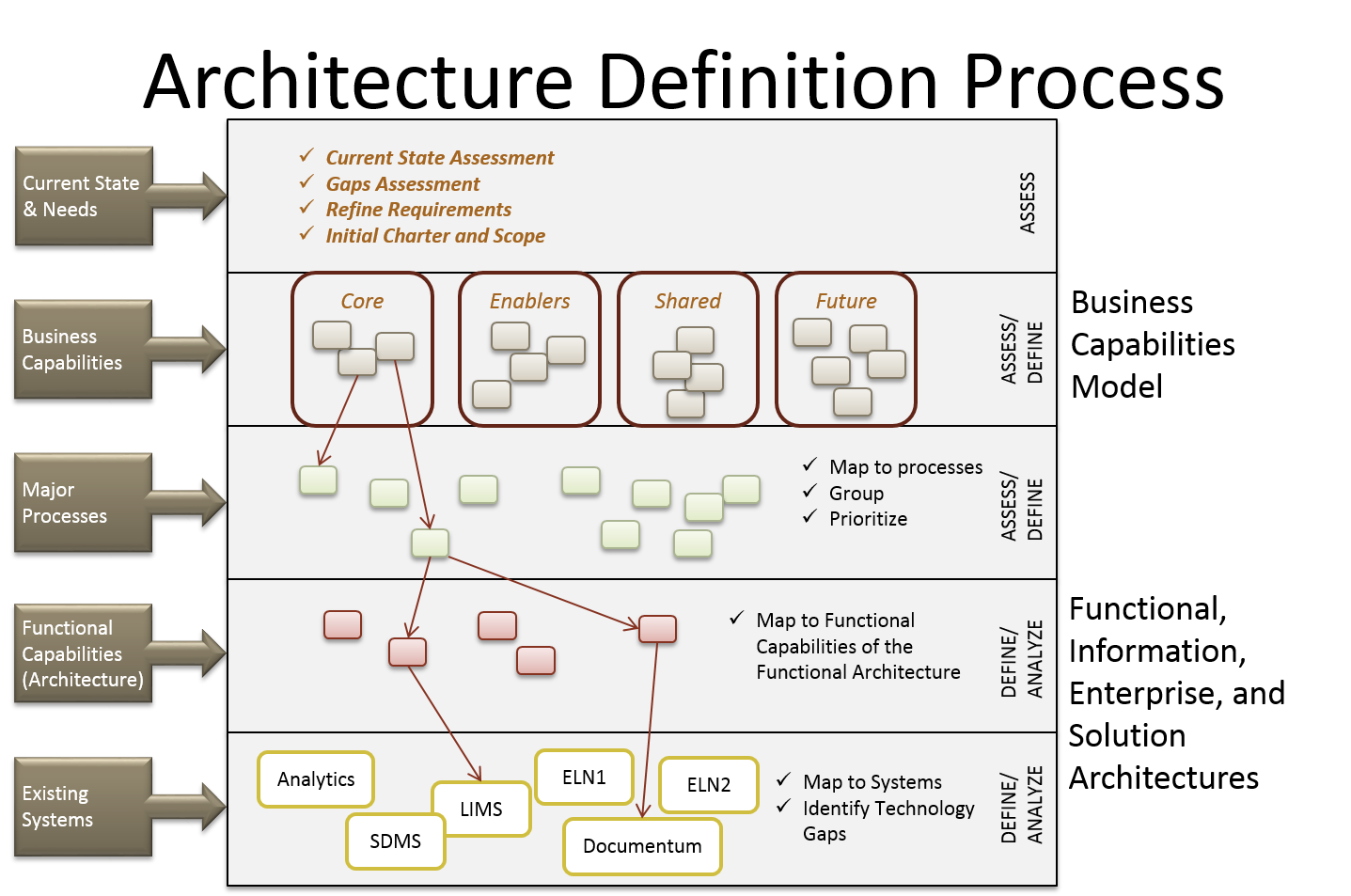
One major issue to overcome was the past delivery of solutions that attempted to solve pieces of the problem. Our architectural analysis approach helped uncover the overly complex and costly to manage solutions that were impeding the client’s knowledge capabilities. The existing governance model was not capable of providing the required support to deliver useful solutions. ArborSys helped the client to design a new model for the program that clearly aligned the value of its use to the business values targeted by the program. Architecture is now positioned to drive the long-term achievement of the program’s goals.
Programs like this require more than just an IT-centric, PMP management framework. ArborSys provided the client with its Enterprise (Process, Programs, Projects) Framework (EP3F©) that combines elements of Process Design and Improvement (Lean and Design for Six Sigma) with PMI and Prince 2. This framework ensures that solutions are developed based on the right approach and not driven by an assumption that technology is always the answer. Change management was recognized as a key need in order to address many entrenched behaviors that need to be replaced in order for any solution to be successful.
ArborSys was able to assemble a team with deep expertise in developing this type of strategic program. We brought together our thought leader in managing content in the life sciences world with senior leaders in business, information, and enterprise architecture to work closely with the client’s dedicated team of experts. Close collaboration is key to ensuring the client can fully own and execute on the detailed program management plans, business requirements, and architectural vision we jointly created.
Value Delivered
The client had a late start to the program due to the length of the decision-making process. ArborSys was able to significantly accelerate the assess and define stages of the program at acceptable risk, based on its approach and the expertise within the team. As a result, the client was able to achieve an understanding of the program’s business case within the budget window required to support approval. This lowered both the cost and risk for the client to proceed.
ArborSys also was able to provide the client with the needed insight and understanding of the 1200 knowledge points discovered in their initial effort. The reduction of this complex map into a high-level set of required capabilities was key to finding a means to communicate what was needed and why. It also provides a manageable controlling framework for delivering on these needs over time. The program is expected to take 4-5 years to achieve its vision. In the past, only short-term thinking was used to create solutions that caused as many problems as they solved. The program management and architectural framework provided allows for managing their long-term vision while ensuring that sufficient short-term gains are achieved to keep the program going. This new capability enables agile management for short-term gain while ensuring the long-term vision is not being impeded. The client will be able to lower its cost of ownership while also increasing the return on its technology assets. Few solutions will be needed and those in place will be leveraged for maximum value. And, most important to the client, their very talented and highly valuable scientific staff will be engaged in doing the work rather than avoiding the work to create, curate, and consume Pharmaceutical Development knowledge.
Want to learn more?
For more information about this case study or any of our services, contact us by email: sales@arborsys.com
Related Content
Related Offerings / Services
- Technology Selection Advisory Services
- Collaboration and Knowledge Management
- Pharmaceuticals Industry
